The Syllabus and Chapter 10 Book Assignments
|
As you are about to discover, radioactivity occurs naturally and is an integrated component of our environment. It's here to stay and it effects our daily lives. Consequently, this chapter is important. You should have a good working knowledge of the chapter concepts outlined in the schedule. In chapter two, you learned about basic atomic structure. This and chapter 11 do their best to break down atomic structure. I guess we have to make it and understand it before we can break it. But, consider: how amazing is it that atoms are stable. In the nucleus of gold for example there are 79 protons. Seventy-nine positively charged species in a tiny dense area. But, we know that like charges repel each other. So why is gold and the other elements stable? Because the rules change. At very small distances, all charged and uncharged species of matter attract each other. Radioactivity occurs when atoms are unstable and decay. During this decay process both matter and energy are released. Which atoms are unstable? Predominately those that don't have the magic number of neutrons binding the protons together. Your text does not emphasize the fact that the decay process releases both matter and energy. The energy can be high or low depending on which part of the electromagnetic spectrum is involved. The electromagnetic spectrum is energy, it is not particulate, it does not contain matter. |
|
Electromagnetic Spectrum |
|||||||
|
¬ Increasing Energy ¬ |
|||||||
|
¬ Increasing Frequency ¬ |
|||||||
|
® Increasing Wavelength ® |
|||||||
|
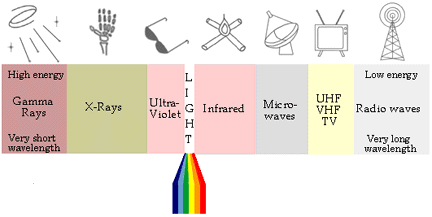
Cosmic rays |
X Rays |
Ultraviolet Rays |
Visible Rays |
Infrared Rays |
Micro waves |
T.V. Rays |
Radio Waves |
|
Violet |
Blue |
Green |
Yellow |
Orange |
Red |
|
It is important to notice that not all decay products are dangerous. It depends on the strength of the energy and the velocity/penetrating power of a particle. Energy emitted from visible to radio waves produces little damage depending on the intensity. The intensity is the number of energy units (photons) hitting a surface per unit time. Bright light as opposed to dim light. Ultraviolet wavelengths are of comparative high energy. These are the wavelengths that affect tanning. They increase the concentration of melanin pigments on the surface of the skin. This is a physiological protection mechanism to prevent high-energy radiation from entering the body. Why do we voluntarily tan ourselves? Decay products come in different masses and different velocities. The beta particle (an electron)for example has little mass and its penetrating power most often depends on its velocity. The alpha particle ( a helium nucleus) is comparatively heavy and most often has a very low velocity. You theoretically could rub it on your skin without damage. But don't drink it or inhale it or the dust particle it might be sitting on. The following on line assignments cover both chapters 10 and 11. They are due June 22. I changed the rules. Is that what rules are for? The "end of chapter" assignments for Chapt 10 are still due on June 15th .
|